Vitamin D Receptor (VDR)
|
VDR is a nuclear receptor which plays a critical role in cell differentiation, proliferation, and calcium homeostasis, and has been identified as an important pharmaceutical target for the treatment of metabolic disorders, skin diseases, cancer, autoimmune diseases, and cardiovascular diseases. VDR is a transcription factor, which is activated by binding to calcitriol (1,25-dihydroxyvitamin D3) and other active metabolites of vitamin D. |
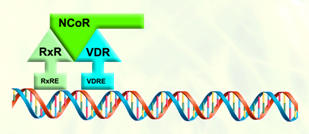
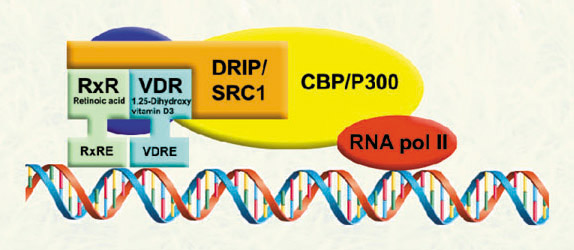
The corepressor are associated with the VDR-retinoid x receptor dimer in the absence of ligand, suppressing VDR-mediated transcription. Ligand binding permits VDR-coactivator interactions, and allows the formation of multi-protein complexes that activate VDR-mediated transcription. |
|
|
The expression of hundreds of genes are regulated by VDR in response to 1,25-dihydroxyvitamin D3. Our investigations are focused on genes directly regulated by VDR in order to understand the physiological role of VDR-coregulator interactions in VDR biology. |
Vitamin D receptor-Coregulator Inhibitors
|
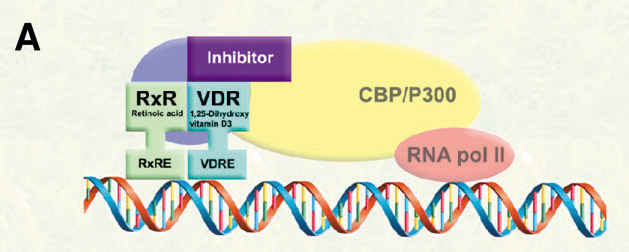
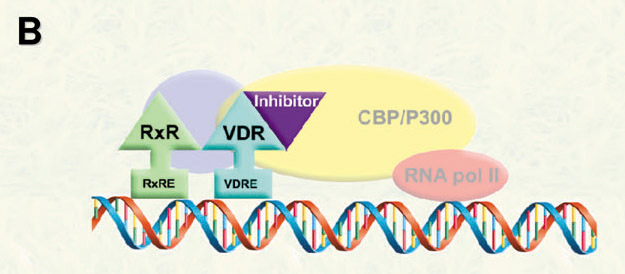
In order to modulate VDR-mediated transcription, we propose the development of small molecules to inhibit the interactions between VDR and coregulators (corepressor and coactivators).
|
First irreversible inhibitors of the VDR-SRC2 interaction
|
|
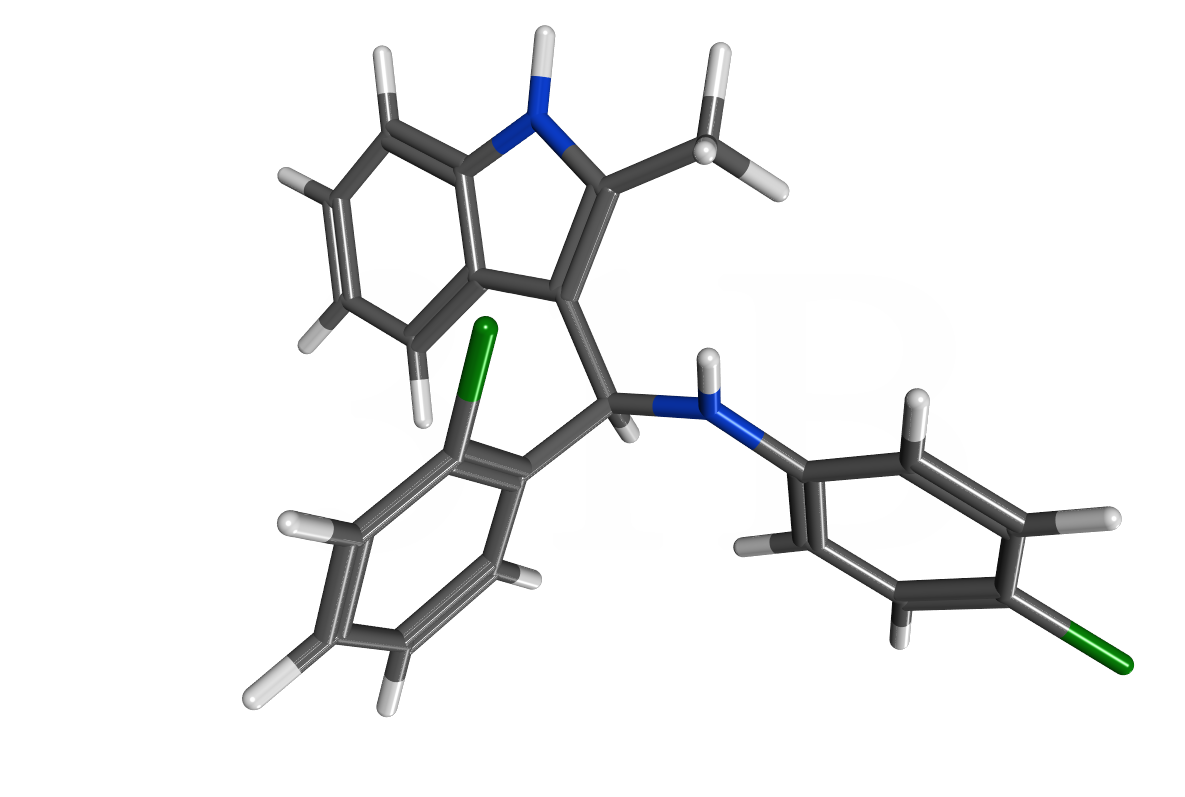
The first discovered irreversible inhibitor of the VDR-SRC2 interaction was 31B published in the Journal of Medicinal Chemistry in 2011. The structure is based on a 3- indoylmethanamine scaffold that enables upon protonation the generation of a electrophilic species that selectively reacts with VDR and inhibit the interaction with coactivators such as SRC2. 31B also modulates the transcriptional response of VDR in the presence of the endogenous ligand 1,25-dihydroxyvitamin D3. Thus the expression of genes responsible to proliferation and differentiation are modulated. Currently, these compounds are investigated in collaboration with Dr. Rakesh Singh (Brown University) in cis-platinum resistant ovarian cancer cells in vitro and in vivo. Recent data show a significant reduction of tumor growth without showing any signs of toxicity or hypercalcemia. 31B is part of a US patent titled Vitamin D Receptor-Coregulator Inhibitors and is one of the first compounds that interact with VDR without modulating calcium homeostasis. |
|
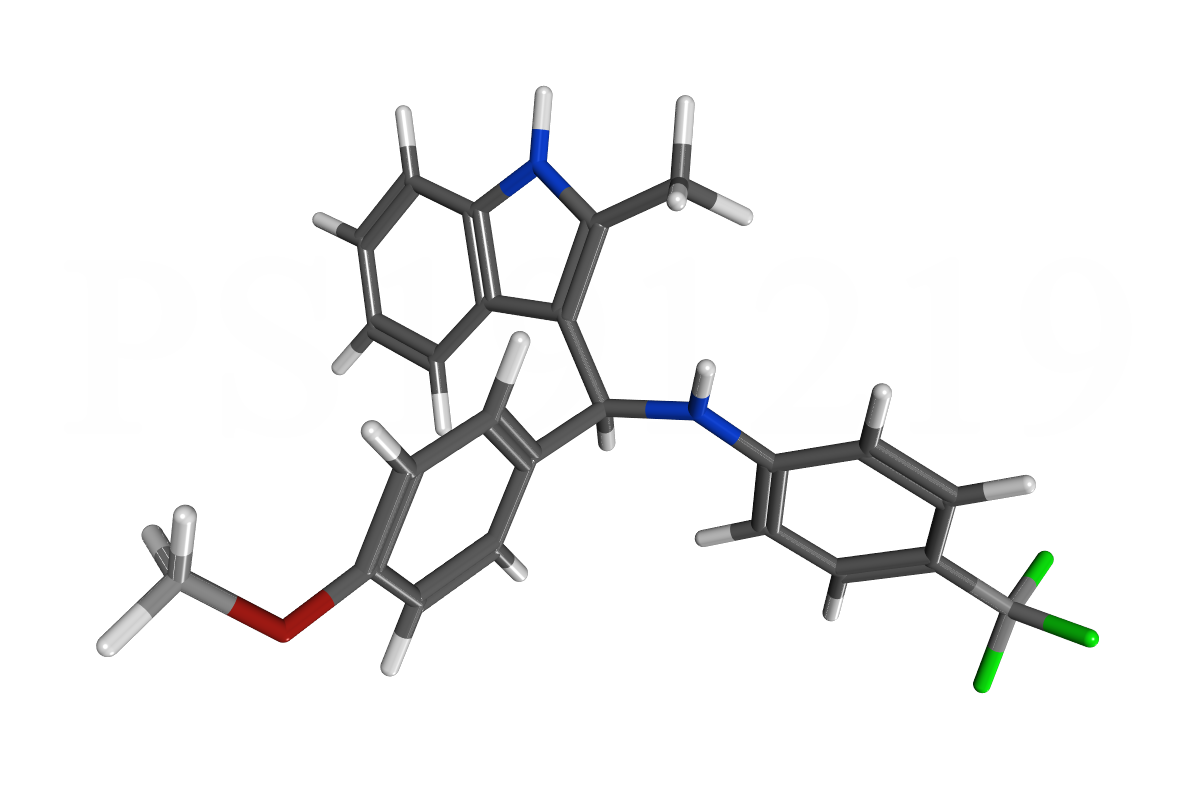 |
PS121912 is a new highly active and selective molecule that inhibits the interaction between VDR and coregulator SRC2. We published the development of this compound in the beginning of this year in ACS Medicinal Chemistry Letters. In addition, we characterized the gene regulation induced by PS121912 in respect to VDR target genes and genes regulating cell proliferation and cell cycle. Especially, P21 and cyclin D1 were identified as mediator of anti-proliferation. The evaluation of PS121912 in respect to its anticancer properties is described in Cancer Chemotherapy and Pharmacology. The compound is very selective in respect to leukemia over other cancers, which prompted us to carry out in vivo xenograft studies with PS121912. Two studies applied different treatment and dosage regimes. In both cases, a significant inhibition of tumor growth was observed. We published these results in Anticancer Research in 2015. |
Reversible inhibitors of the VDR-SRC2 interaction
|
|
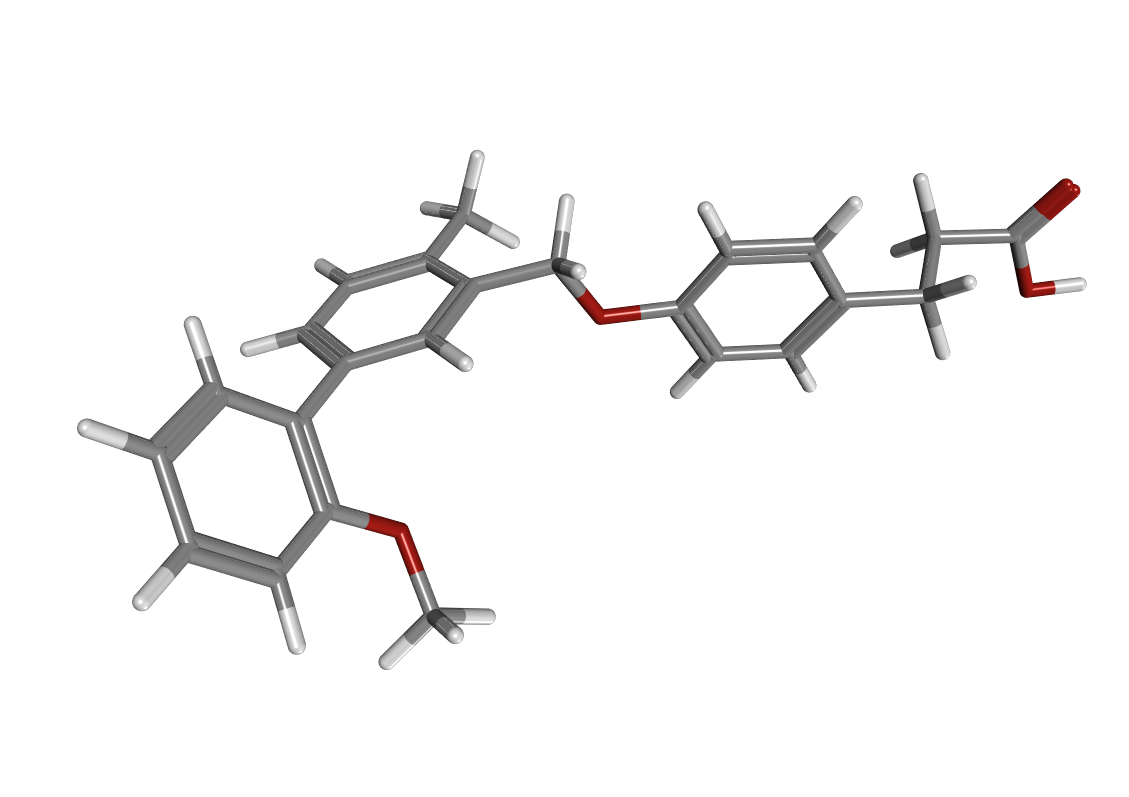
Earlier last year we discovered that GW0742, a peroxisome proliferator-activated receptor gamma agonist, can inhibit the interaction between VDR and SRC2 at higher concentrations (Biochemistry, 2013). Based on these results, we have been working very closely together with my collaborators at the NIH (NCGC, Drs. Maloney, Jadhav, and Simeonov). Their medicinal chemistry core facility synthesized more than a hundred compounds for us that were subsequently analyzed by Kelly Teske using biochemical and cell-based assays. In addition, my graduate student Belaynesh Feleke synthesized a different series of GW0742 analogs and discovered a more potent and more selective VDR-SRC2 inhibitor. We are currently crystallizing the vitamin D receptor with these compounds to identify the mode of binding. This work is carried out in collaboration with Dr. Silvaggi (UWM) and his graduate student LanLan Han. Some earlier results were published in the Journal of Biomolecular Reseach & Therapeutics in 2014. |
 |
|
Based on a high throughput screen conducted with the NIH in 2010, a molecular probe developing plan was accepted earlier this year by the NIH and the National Center of Advancing Translation Science (NCATS) in order to support medicinal chemistry efforts by NCATS and the Kansas Chemistry of Excellence Center (KU). Both research groups are currently synthesizing new molecules around a novel scaffold in order to identify molecular probes for the VDR-coregulator interaction to be used in vivo. Independently, an R01 application has been submitted to the NIH to further support this effort by our group. The group effort has made excellent progress and a manuscript is in the process. |